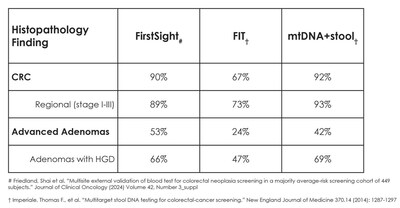
CellMax Life, a molecular diagnostics company with proprietary technology for pre-cancer and cancer detection blood tests, presented the latest results from its independent external validation study on January 20, 2024 at the ASCO Gastrointestinal Cancers Symposium. Data from this external validation study showed that FirstSight™ was able to detect CRC with 92% sensitivity and advanced adenomas (AA) with a 53% sensitivity, increasing to 66% for adenomas with high-grade dysplasia and 69% for large adenomas ≥3cm in size. These results demonstrate best-in-class performance and increase in significance when compared to stool tests and first-generation liquid biopsy tests.
SUNNYVALE, Calif., Jan. 26, 2024 /PRNewswire-PRWeb/ -- CellMax Life, a molecular diagnostics company with proprietary technology for pre-cancer and cancer detection blood tests, presented the latest results from its independent external validation study on January 20, 2024 at the ASCO Gastrointestinal Cancers Symposium.
The study used a predefined and locked assay, algorithm and clinical threshold developed from the previous N1038 model development study to evaluate the performance of FirstSight™ in an independent external validation set with subjects not previously tested. A demographically representative, majority intended-use average risk cohort of 449 subjects across 15 sites in the U.S. and 1 site in Canada were analyzed in the external validation set.
Data from this external validation study showed that FirstSight™ was able to detect CRC with 92% sensitivity and advanced adenomas (AA) with a 53% sensitivity, increasing to 66% for adenomas with high-grade dysplasia and 69% for large adenomas ≥3cm in size. These results demonstrate best-in-class performance and increase in significance when compared to stool tests and first-generation liquid biopsy tests.
"There is a significant unmet clinical need when it comes to detecting colorectal cancer and advanced adenomas," said Shai Friedland, MD, lead principal investigator on the study and gastroenterologist at Stanford Medicine. "The challenges come because stool tests and alternative options such as routine colonoscopies have low compliance. The results from this external validation study are incredibly promising; a blood test that can detect early-stage colorectal cancer and advanced adenomas with high sensitivity along with vastly improved screening rates has the potential to pre-empt progression to cancer."
The FirstSight™ blood test is uniquely different from all other ctDNA blood tests and is able to detect advanced adenomas by combining molecular and cellular signatures not just from ctDNA but also from rare epithelial cells shed from adenomas using the company's proprietary circulating epithelial cells (CEC) technology.
"There are too many unnecessary deaths from colorectal cancer," said Atul Sharan, co-founder and CEO of CellMax Life. "Our goal with FirstSight™ continues to be to provide a screening blood test which has not only high sensitivity for colorectal cancer, but also improved detection of advanced adenomas to facilitate targeted colonoscopies for removal and prevent cancer, to the approximately 50 million people who don't follow the American Cancer Society guidelines for screening."
Data from this study, and from previous publications and abstracts, continues to highlight FirstSight's™ ability to detect both CRC and advanced adenomas, and suggests results would be reproducible in a large clinical study. CellMax received the expedited pathway Breakthrough Device Designation from the U.S. Food and Drug Administration in Q3 2021 for FirstSight™.
The company plans to begin a PMA study for an advanced adenoma-only test that the FDA may consider as an adjunct to guideline-approved CRC screening tests to improve CRC prevention. If approved, the highly sensitive, noninvasive FirstSight™ blood test will help increase routine screening compliance for patients who are reluctant to undergo a colonoscopy or stool testing and may help prevent colorectal cancer by detecting the disease earlier when treatment success and survival rates are much higher.
About CellMax Life
CellMax Life is a molecular diagnostics company focused on cancer screening with proprietary technology for detecting precancerous and cancer cells and genomic aberrations in a single blood sample. CellMax Life is headquartered in Sunnyvale, California. For more information, visit http://www.cellmaxlife.com.
Media Contact
Stephen Su, CellMax Life, 1 408-359-7819, [email protected], www.cellmaxlife.com
SOURCE CellMax Life


Share this article